P637 Chegg Consider the Continuous Bio Reactor
| Figure 11.9 Different arrangements and modes of operation for membrane bioreactors Continuous Stirred Tank Reactor (CSTR) with recirculation arrangement (a), dead-end cell (b), tubular with entrapped enzyme (c). | 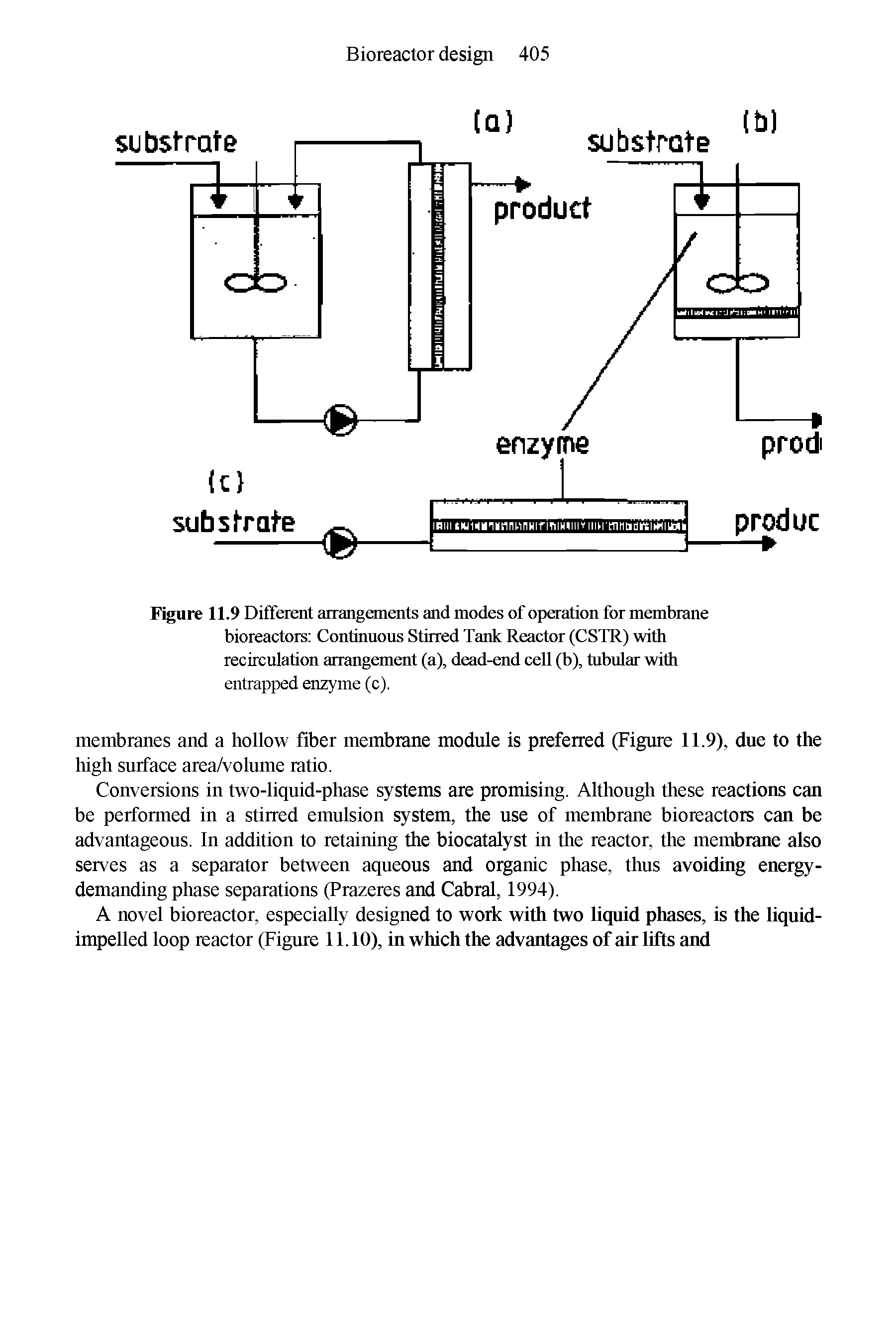 |
Continuous SSF processes are usually operated in plug flow mode. Such processes will require pasteurization or sterilization of the substrate as it enters the bioreactor, mixing with an inoculum, and at the outlet end of the bioreactor, continuous removal of spent substrate. Such a process was operated on a pilot scale for the production of ethanol from fodder beets by Saccharomyces cerevisiae [81,82]. The bioreactor had a screw within a 4.7 m long and 15.25 cm diameter tube. The screw was rotated intermittently to mix the substrate and move it along the tube. At the front end was a hammer-mill and a pasteurization chamber for substrate preparation and a port for inoculation. New substrate was added, inoculated, and the screw rotated at 12-h intervals, resulting in a residence time of 72 h. [Pg.100]
Relative to stricfly batch bioreactors, continuous and fed batch bioreactors require more connections to process stteams through which sterility must be maintained throughout the entire period the reactor is onstream. When contamination does occur in equipment used to carry out a continuous process, it can rapidly spread to downstream units. By contrast, confinement or isolation of contaminants can be accomplished more readily in a batch reactor and its associated auxiliary facilities. [Pg.481]
Laska ME, Cooney CL. (2002) Bioreactors, continuous stirred-tank reactors. In FUckinger MC, Drew SW, editors. Encyclopedia of Bioprocess Technology, John Wiley Sons, Inc., New York, USA. p 353-371. [Pg.309]
Cells in suspension Plug flow reactor with hydrodynamic stirring with or without static mixing elements in tube or flat-bed bioreactor Continuous... [Pg.11]
Single-Cell Protein. Systems involving single-cell proteins are often very large throughput, continuous processing operations such as the Pmteen process developed by ICI. These are ideal for air-lift bioreactors of which the pressure cycle fermenter is a special case (50). [Pg.337]
Production of the vims in a bioreactor reactor, using a continuous cell line, has also been studied (85,86). This will reduce production costs and side effects. Both Madin-Darby canine kedney (MDCK) and Vero cell lines are being developed for production of the vaccine. [Pg.359]
Alternatively, some subunit viral vaccines can be generated by rDNA techniques and expressed in a continuous ceU line or insect ceUs. Recent advances in bioreactor design and operation have improved the successful production of IPV in large-scale bioreactors. However, roUer bottles or flasks are stiU used for most current vaccine production. Development of insect ceU culture will allow for very large-scale Hquid suspension culture (143). Several vaccine candidates such as gpl60 for HIV and gD protein for herpes have been demonstrated in the insect ceU culture system. However, no vaccine has been approved for human use. [Pg.361]
Stuckey, D.C., Caridis, K.A., Leak, D.J., Design of a novel bioreactor with cell recycle for continuous biotransformation and product extraction, Proc. 3rd Asia Pacific Biochemical Eng. Conf, Singapore, pp.315-317, 1994. [Pg.368]
Bioreactors a) batch stirred tank b) continuous stirred tank c) continuous packed-bed i) downward flow, ii) upward flow and iii) recycle d) continuous fluidised-bed e) continuous ultrafiltration. Redrawn from Katchalski - Katzir E. (1993) Trends in Biotechnology II, 471-477. [Pg.16]
In Japan Chlorella spp has been produced for food in continuous aseptic systems in conventional bioreactors. The organisms are grown in the dark as heterotrophs using sucrose (in the form of molasses) or glucose as carbon and energy source. Production has been 2,000-3,000 tonnes per year at a selling price of (US)10-22 kg 1 (1990 prices). This product is sold as a high-value health food. [Pg.73]
Item 1 is the major factor in choosing continuous systems rather than batch systems for improved SCP production. Economics are improved by lower capital cost for the bioreactor (a conomica major equipment cost, see Section 4.10) and by a higher output rate. Item 3 leads to greater control of product quality. [Pg.92]
The production-scale fermentation unit, with a projected annual capacity of over50,000 tonnes was fully commissioned in 1980. The bioreactor (Figure 4.8) is 60 m high, with a 7 m base diameter and working volume 1,500 m3. There are two downcomers and cooling bundles at the base. Initial sterilisation is with saturated steam at 140°C followed by displacement with heat sterilised water. Air and ammonia are filter sterilised as a mixture, methanol filter sterilised and other nutrients heat sterilised. Methanol is added through many nozzles, placed two per square metre. For start-up, 20 litres of inoculum is used and the system is operated as a batch culture for about 30 h. After this time the system is operated as a chemostat continuous culture, with methanol limitation, at 37°C and pH 6.7. Run lengths are normally 100 days, with contamination the usual cause of failure. [Pg.100]
The submerged culture process continues to increase in terms of the percentage of dtric acid produced compared to that produced by the surface culture method. Tower bioreactors are preferred over stirred reactors because they cost less, there is less risk of contamination and they are less limited by size. [Pg.135]
A full set of bioreactors with pH and temperature controllers are shown in Figure 1.3. The complete set of a 25 litre fermenter with all the accessory controlling units creates a good opportunity to control suitable production of biochemical products with variation of process parameters. Pumping fresh nutrients and operating in batch, fed batch and continuous mode are easy and suitable for producing fine chemicals, amino acids, and even antibiotics. [Pg.12]
The mass transfer, KL-a for a continuous stirred tank bioreactor can be correlated by power input per unit volume, bubble size, which reflects the interfacial area and superficial gas velocity.3 6 The general form of the correlations for evaluating KL-a is defined as a polynomial equation given by (3.6.1). [Pg.45]
| Fig. 4.1. Instrumentation control for continuous stirred tank (CSTR) bioreactor. | 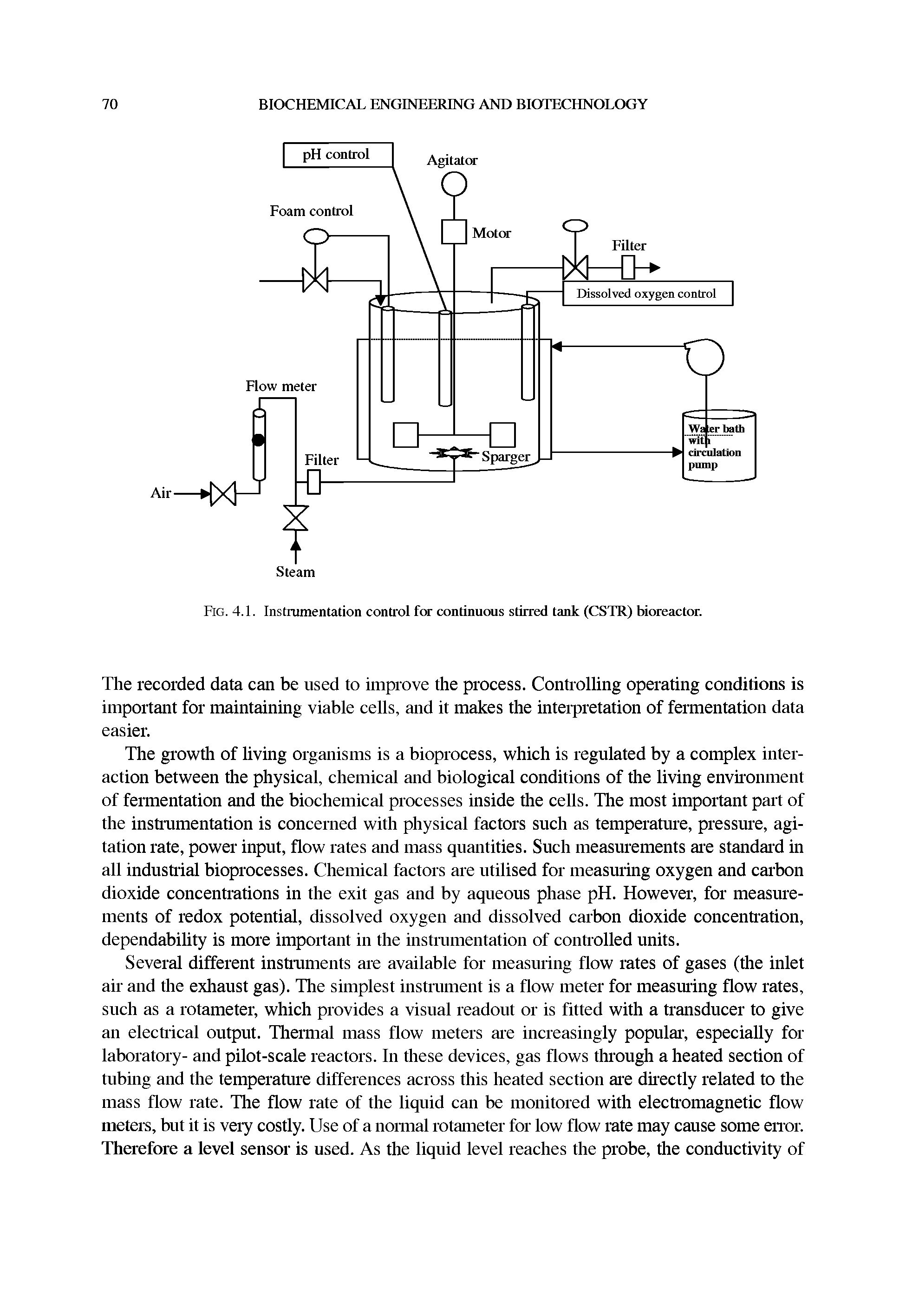 |
Batch mixed reactor There are three principal modes of bioreactor operation (a) batch (b) fed batch (c) continuous. [Pg.144]
In a batch fermentation of ethanol, kinetic data were collected as product formed. The data are shown in Table E.5.1. The data will be used to design a continuous bioreactor (CSTR) with a 1001 working volume. [Pg.320]
Advancing the field of process engineering. Important generic goals for research include the development of separation processes for complex and fragile bioproducts the design of bioreactors for plant and mammalian tissue culture and the development of detailed, continuous control of process parameters by rapid, accurate, and noninvasive sensors and instruments. [Pg.15]
Source: https://chempedia.info/info/bioreactors_continuous/
0 Response to "P637 Chegg Consider the Continuous Bio Reactor"
Post a Comment